Decoding Dysautonomia, Part 2: The Long COVID Connection
The Underlying Causes No One’s Talking About
Long COVID: More Than Just Lingering Symptoms
At first, it felt like just another cold. But what came next was anything but normal. Like so many others, my long COVID story follows a frustratingly familiar pattern. I had a mild case of COVID in January 2023, but shortly after recovering, things took a turn.
My heart rate spiked for three days between 100-160 BPM at rest, leaving me with chest pain, tremors, and crushing fatigue. A trip to the ER revealed several abnormal labs but no clear answers. After stabilizing my heart rate with an IV and electrolytes, I was told I had long-COVID and sent home to recover.
But that was only the beginning. What I thought was a one-time ordeal turned into an ongoing cycle of unpredictable flare-ups, reshaping my daily life in ways I never expected.
I later learned that I was far from alone. Estimates suggest that 10-35% of people who get COVID develop lingering symptoms that can persist for months or even years. Even more striking, research shows that nearly 90% of long-COVID cases occur after mild infections, meaning a severe initial illness isn’t necessary for long-term complications to arise.
Know someone struggling with long COVID? Share this post with them:
While anyone can develop long COVID, research suggests that women face a significantly higher risk, with studies estimating they are 31% more likely to develop the condition than men. Though researchers are still uncovering why women are at higher risk, one thing is clear—long COVID is anything but predictable in how it presents. Many cases follow a similar pattern; however, the range of symptoms is vast. Over 200 symptoms have been reported, affecting nearly every system in the body.
This is what makes long COVID so complex. It doesn’t just impact one system—it can affect nearly every organ. And a big reason for this is how the virus gains entry in the first place.
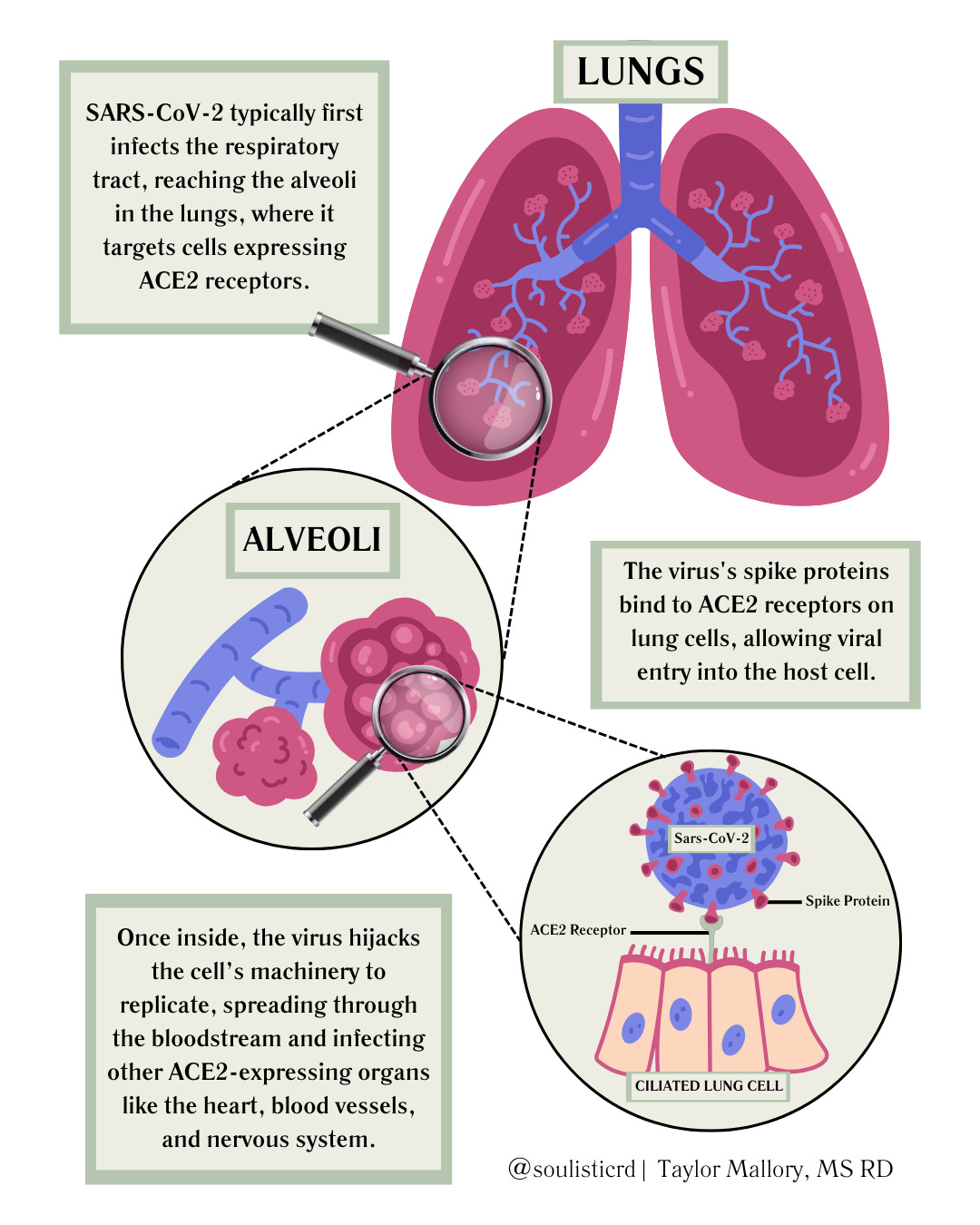
How COVID-19 Hijacks the Body: The Role of ACE2 Receptors
We often think of COVID-19 as just a lung infection, but in reality, it’s more like a cellular hijacker. SARS-CoV-2 (the virus that causes COVID-19) doesn’t break down the door by force—it’s sneakier than that. Its spike proteins act like a forged keycard, tricking ACE2 receptors—proteins that normally help regulate blood pressure, inflammation, and fluid balance—into letting it in. Once inside, the virus hijacks the cell’s machinery, forcing it to churn out copies of itself and spread the infection.
ACE2 receptors aren’t just hanging out in the lungs. They’re everywhere—in the heart, blood vessels, kidneys, muscles, gut, and nervous system. This explains why COVID-19 doesn’t just stop at the respiratory system. The image below illustrates how the virus first infects the lungs before spreading to other ACE2-rich tissues throughout the body:
As the virus depletes ACE2 receptors across multiple organ systems, it interferes with key regulatory processes, making it harder for the body to maintain stability. This widespread dysfunction can set the stage for dysautonomia by impairing the body's ability to regulate heart rate, blood pressure, body temperature, and more. If you’re new to the term, I covered what dysautonomia is, its symptoms, and how it impacts the body in Part 1 of Decoding Dysautonomia.
While the exact mechanisms are still being studied, research suggests that RAAS dysregulation, mitochondrial dysfunction, endothelial damage, and vagus nerve inflammation may play a role—each of which we'll explore next.
The Root Causes of Long COVID Dysautonomia
Long COVID-related dysautonomia is still being actively researched, and there isn’t a single definitive answer as to what causes it. However, current evidence suggests that several mechanisms may contribute to autonomic nervous system dysfunction. For some individuals, one of these factors may be the primary driver, while for others, multiple overlapping dysfunctions create a perfect storm. Based on emerging research, here are some of the strongest proposed mechanisms:
RAAS Dysregulation: A Fluid Imbalance
Remember those ACE2 receptors we just talked about? They’re not only entry points for the virus—they also play a key role in regulating the renin-angiotensin-aldosterone system (RAAS), which helps control blood pressure, vascular tone, and fluid balance. When SARS-CoV-2 hijacks ACE2 receptors, pulling them into the cell, it disrupts RAAS and throws off the body's ability to maintain stable blood pressure and volume.
The Renin-Aldosterone Paradox
RAAS normally responds to low blood volume by triggering the release of renin (from the kidneys) and aldosterone (from the adrenal glands). Aldosterone helps the body hold onto sodium and water, increasing blood volume and stabilizing blood pressure.
But in long COVID and hypovolemic POTS (a form of dysautonomia characterized by low blood volume), something strange happens:
Renin levels are lower than expected for someone with low blood volume.
Aldosterone is too low, meaning the body isn’t retaining enough sodium and fluids.
This is known as the renin-aldosterone paradox—instead of correcting the problem, the body fails to compensate, leading to persistent low blood volume (hypovolemia).
Why Does This Matter?
Since low blood volume = less oxygen delivery to the brain and muscles, this imbalance contributes to many hallmark symptoms of dysautonomia and long COVID, including:
Dizziness and lightheadedness upon standing
Rapid heart rate (tachycardia)
Blood pressure swings
Fatigue and exercise intolerance
Because the body struggles to retain fluids, increasing salt and fluid intake is often recommended for POTS patients to help restore blood volume and improve symptoms.
RAAS dysfunction is just one piece of the puzzle—next, we’ll explore how mitochondrial dysfunction, endothelial damage, and vagus nerve inflammation may play a role in long COVID-related dysautonomia.
Mitochondrial Dysfunction: The Energy Crisis
Your autonomic nervous system (ANS) has a demanding job—it regulates heart rate, blood pressure, digestion, and body temperature, all without you thinking about it. But to do this, it needs a steady supply of energy. That’s where mitochondria come in.
Mitochondria: The Powerhouse of the Cell
Mitochondria are tiny cellular power plants responsible for converting nutrients from food into ATP, the body’s main energy source. When they’re working efficiently, they fuel everything from muscle contraction to brain function.
But in long COVID, something disrupts this process.
Mitochondrial Breakdown in Long COVID
This disruption weakens mitochondrial function, reducing their ability to generate energy. Researchers have identified several ways this breakdown occurs:
Direct mitochondrial damage—SARS-CoV-2 may directly impair mitochondrial function, limiting their ability to generate ATP.
Inflammation interference—Chronic inflammation disrupts mitochondrial efficiency, further reducing energy production.
Oxidative stress overload—Excess free radicals (unstable molecules) outpace the body’s ability to neutralize them, causing cell and mitochondrial damage.
This combined strain on the mitochondria leads to ATP shortages, meaning the ANS and muscles don’t get the energy they need. As a result, symptoms like fatigue, poor temperature regulation, and exercise intolerance emerge.
More Than Just Energy: Mitochondria & Metabolism
Beyond energy production, mitochondria play a crucial role in metabolism, influencing how the body processes and utilizes nutrients. When mitochondrial function is impaired, disruptions in metabolic pathways can lead to imbalances that affect weight regulation, energy levels, and nutrient absorption—potentially resulting in unexplained weight changes and deficiencies over time. This highlights the importance of nutrient testing as a tool for identifying targeted interventions in long COVID dysautonomia.
Energy production and metabolism are just scratching the surface—blood vessels are also deeply affected. Mitochondrial dysfunction and oxidative stress can contribute to endothelial damage, further disrupting circulation and vascular health. Next, we’ll explore how this breakdown impacts long COVID and dysautonomia.
Endothelial Damage: A Circulatory Breakdown
The Endothelium: More Than Just a Lining
Blood vessels do more than transport oxygen and nutrients—they actively regulate circulation and vascular health. This regulation is largely controlled by the endothelium, a thin layer of cells lining the inside of all blood vessels. It plays a critical role in maintaining blood flow, preventing clotting, and modulating inflammation.
The Glycocalyx: A Protective Shield
Lining these endothelial cells is the endothelial glycocalyx, a gel-like layer that serves as a crucial protective barrier. More than just structural support, the glycocalyx helps regulate vascular permeability, inflammation, and the production of nitric oxide (NO)—a molecule essential for blood vessel dilation.
However, research shows that SARS-CoV-2 can degrade the glycocalyx, leading to endothelial dysfunction, increased clotting risk, and impaired oxygen delivery. When this protective layer breaks down, small blood vessels (microvasculature) struggle to supply oxygen to the brain, muscles, and organs.
In the brain, glycocalyx breakdown can also compromise the blood-brain barrier—a protective network of blood vessels that tightly regulates which substances gain entry. Think of it as the brain’s VIP security, deciding who gets access and who gets turned away. When this barrier becomes more permeable, inflammatory molecules can slip past its defenses, contributing to neurological impairments such as cognitive dysfunction and neuroinflammation.
The Consequences: Long COVID Symptoms
Remember how glycocalyx degradation increases clotting risk? This breakdown can lead to the formation of microclots, which clog capillaries, prolong oxygen deficits, and fuel ongoing inflammation. Some researchers also suggest that microvascular impairment may explain persistent low oxygen levels in certain long COVID patients, even when lung function appears normal.
The combination of impaired oxygen delivery, increased clotting risk, and neuroinflammation has been linked to common long COVID and dysautonomia symptoms, including:
Shortness of breath
Chest pain
Brain fog
Fatigue
The Link to Dysautonomia & POTS
Many of these symptoms closely align with postural orthostatic tachycardia syndrome (POTS), a form of dysautonomia commonly seen in long COVID. Endothelial dysfunction can lead to poor circulation and difficulty regulating blood pressure and heart rate, particularly during positional changes. This may explain why some individuals with POTS experience lightheadedness, rapid heart rate, and blood pooling in the lower extremities upon standing.
These vascular changes highlight how endothelial and glycocalyx damage contribute to the lingering effects of long COVID and its overlap with dysautonomia.
As you can see, there are many moving parts to decoding dysautonomia. The final piece of the puzzle ties in the notorious vagus nerve—another key player in autonomic regulation and long COVID-related dysfunction.
Vagus Nerve Inflammation: The Misfiring Nervous System
The vagus nerve is the body's longest and most complex nerve, acting as the main communication highway between the brain and vital organs like the heart, lungs, and gut. Think of it as the body's internal fiber-optic network—relaying crucial messages that keep automatic functions running smoothly. It regulates:
Heart rate and blood pressure – maintaining cardiovascular stability
Breathing patterns – regulating respiratory rhythm and diaphragm function
Digestion and gut motility – coordinating the movement of food through the digestive tract
Inflammation and immune response – helping to regulate the body's defense system
But what happens when this communication highway gets disrupted?
How SARS-CoV-2 Disrupts the Vagus Nerve
Emerging research suggests that SARS-CoV-2 can infect and inflame the vagus nerve, throwing its finely tuned signaling off balance. This inflammation disrupts vital autonomic functions, triggering a cascade of symptoms:
Erratic heart rate and blood pressure swings, leading to dizziness and palpitations (POTS-like symptoms)
Air hunger, a distressing sensation of not getting enough air despite normal oxygen levels
Nausea, abdominal pain, and digestive issues due to impaired gut-brain signaling
Over time, this dysregulation can trap the nervous system in a chronic fight-or-flight state, fueling ongoing autonomic instability and making recovery even more challenging.
Restoring Balance Through Nervous System Regulation
Because the vagus nerve plays such a critical role in autonomic stability, nervous system regulation is an important piece of the recovery puzzle. Techniques such as breathwork, humming, meditation, the Valsalva maneuver, and others may help stimulate the vagus nerve and promote a parasympathetic (rest-and-digest) response. These strategies and others will be explored in more detail in Part 3, where we’ll discuss nutrition and lifestyle approaches to managing long COVID dysautonomia and supporting recovery.
Key Takeaways & What’s Next
Long COVID-related dysautonomia isn’t caused by a single factor—it’s the result of widespread dysfunction across multiple systems. From RAAS dysregulation to mitochondrial energy deficits, blood vessel damage, and vagus nerve inflammation, these disruptions create a perfect storm for autonomic imbalances. This explains why symptoms like fatigue, exercise intolerance, blood pressure instability, heart rate fluctuations, and others are so persistent in those with long COVID.
I know this firsthand—my own journey with long COVID dysautonomia was anything but linear. The symptoms were unpredictable, fluctuating without clear patterns, making it hard to tell if I was truly improving. There were setbacks, frustrations, and moments where recovery felt impossible.
What’s the biggest challenge you’re facing navigating long COVID or dysautonomia?
Starting any health journey is difficult, but building a strong foundation is key. Without addressing the basics—nutrition, sleep, movement, and nervous system regulation—the next-level strategies are far less effective.
Once I had that foundation in place, I was able to take things a step further. Functional labs helped me uncover nutrient deficiencies and deeper dysfunctions, allowing me to make targeted interventions that supported my recovery. From optimizing mitochondrial and endothelial health to regulating my nervous system, every piece of the puzzle mattered.
Understanding these root causes is just the first step. In Part 3, we’ll explore science-backed nutrition and lifestyle strategies to manage symptoms and restore balance—giving your body the tools it needs to heal.





I wish I had found this comprehensive overview months ago when I really began researching Long COVID and longing for answers to explain what I was going through so I had some game plan of how to start healing. I have found that addressing many of the items you have listed here to be really helpful in my journey too. I’m currently a little over 9 months into my journey with Long COVID myself.
I got dizzy out of the blue one day and thank goddess for “FB Doctors” who diagnosed me in seconds. Since then I’ve been dizzy and can longer live the way I used to. Luckily I eventually stopped grieving and got accustomed to the new me. But I still wonder, how can you be fine one day and the next you’re disabled for life? Its strange.